
Whether you run a small business or a global enterprise, everything you produce reflects on your company’s identity. This includes your product packaging and labels. Unless your brand’s colors are…
Where the finest products along with everything you need for “do-it-yourself” label printing are just a click away.
Call Now: +1-866-299-0066 or Live Chat

Hand sanitizer is regulated by the U.S. Food and Drug Administration (FDA). Due to this regulation, a hand sanitizer label must meet regulatory compliance standards.
To avoid costly fines and possible business shutdown, you should know what’s required on a product label before beginning to market and sell hand sanitizer.
Get the full list of product label requirements based on the FDA regulations and ensure your label maker can meet these requirements.

Hand Sanitizer Label Requirements from the FDA
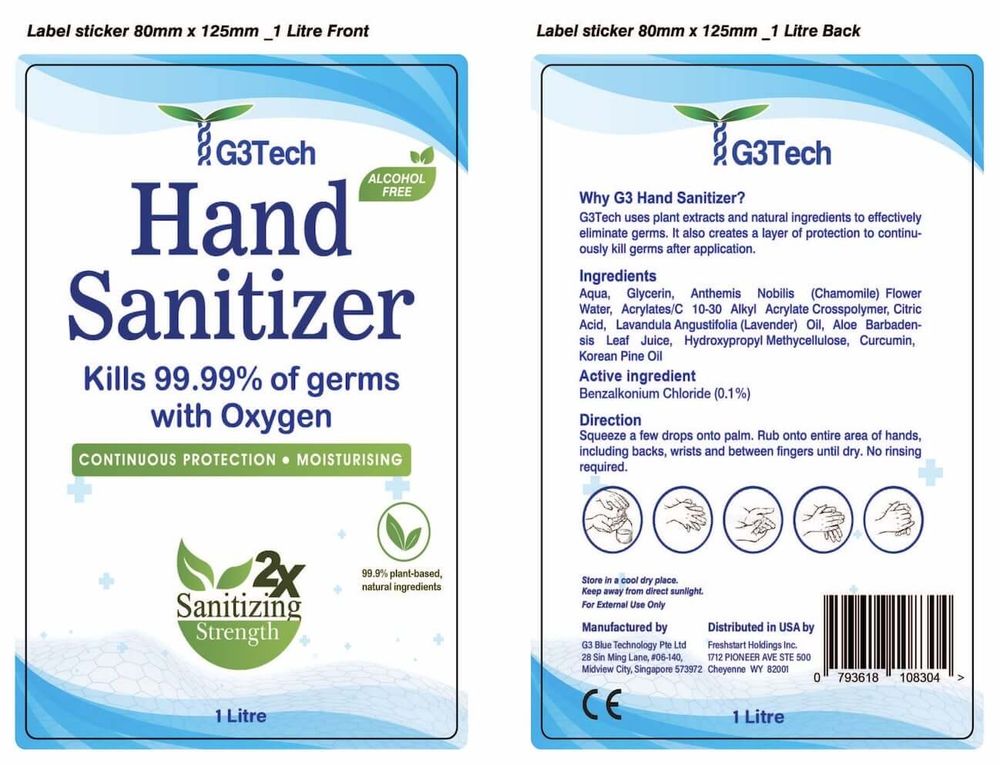
On the surface, hand sanitizer seems like such a simple product to market and sell. But much like food labels, you’re required to include specific information on your hand sanitizer labels.
Hand sanitizers are not traditional drugs, which is why many people are surprised to find that these products fall under the FDA requirements. However, according to the FDA, hand sanitizer is an over-the-counter (OTC) drug product. And as an OTC product, you’re required to meet several regulations to be permitted to sell this product.
When selling an over-the-counter drug product, the FDA outlines several things that you must include on your labels. But the administration also outlines many items you cannot include on your labels. Some pieces of required information also must go on specific areas of the product label, according to the outlined requirements.
The FDA outlines three main areas of important information on hand sanitizer labels.
To be eligible for sale, hand sanitizer products must include the name and business address of the product manufacturer, distributor and packer. This goes on the information panel of the product.
Ideally, you should also put your business or brand name on the front of the bottle so that consumers can identify it quickly and choose it from the busy store shelves where they shop. This will help your product stand out from the competition and allow consumers to select your product again and again when fulfilling their shopping list.
This is the panel that consumers inspect when considering purchasing your product. It is the part of the product that sits facing outward on display for sale. The principal display panel (PDP) must be large enough to include all required information in a readable format. You cannot obscure any important information when designing the PDP despite how much information you must include.
The size of your label will vary depending on the container you select for your hand sanitizer product. But the FDA does outline the following requirements for the PDP.
In addition to requirements for how large the PDP must be and where it must be located on various container types, the FDA requires that this part of the labeling include a statement of identification and the product’s net contents.
This simply states what the product is. That means you must state clearly that the product is hand sanitizer. Not only is this a good practice to meet regulatory compliance, but you’ll want consumers to be able to identify your product quickly.
You must prominently display the product’s net contents in milliliters on the front panel of your product. It’s wise to also include fluid ounces as many people in the U.S. are more familiar with this weight measurement.
The decimal points in your milliliter measurement must not extend past two places. Your net contents should be listed on the bottom 30 percent of the PDP parallel to the container’s base. However, if your PDP is 5 inches or less, this requirement does not apply.
Typically, the drug facts panel is attached to the backside of your product’s container. If you sell the bottle of hand sanitizer inside of other packaging, the drug facts must appear both on the bottle and on the outside packaging.
You cannot put your company or product name on the drug facts panel anywhere and must adhere to strict panel layout and information presentation. The FDA requires that your drug facts panel appear with the following headings in this order.
After you’ve satisfied these requirements, you can add a questions and comments section. This is completely optional though.
Even once you’ve met these regulations for organizing your drug facts label, you’ll need to ensure it also meets the following design requirements.
Each heading must use a uniform and easy-to-read typeface. It should be 39 characters per inch or less. All titles and headings must appear in bold, italic font and no smaller than 8-point font. Subheadings should only be bold and be no smaller than 6-point font.
Although this list provides a good starting point for meeting regulatory compliance for hand sanitizer labels, it is not a substitute for legal advice. You should seek an attorney that specializes in FDA regulations who can review your product labels before you begin printing and using them to market and sell your product.
Because hand sanitizers require both front and back labels of a decent size, the cost of creating these labels can get quite pricey. Before beginning the design process, consider these tips for reducing the expense of labeling these products.
Start printing your own labels with a simple, hassle-free label maker from DuraFast Label Company. We help businesses of all types and sizes select the best label maker for their needs. You can buy or lease a label printer. Our team matches your needs and budget with label printers that use the best ink cartridges at affordable costs to manage the long-term expense of the printer.
Browse our products today to learn more, or contact us for answers to your questions about purchasing a label printer.
Further Reading: